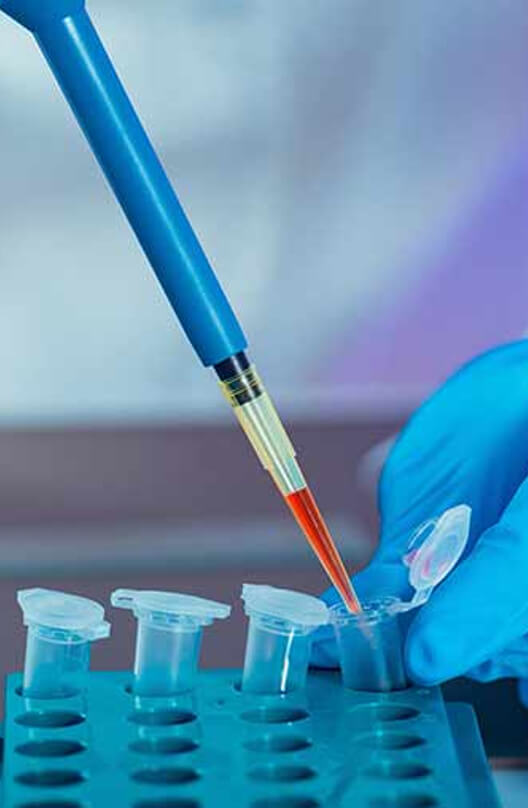
About Company
SHLOK TECHNO is Gujarat based Pharmaceutical consulting firm. Our teams of experts providing a wide range of consulting services to Pharmaceutical & allied healthcare industries like Drug Formulation & Bulk Drug (API), Paramedical Herbal Phytochemicals and Neutraceuticals. This team of professionals is well positioned to help you identify and enhance business opportunities in the rapidly growing International markets.
SHLOK TECHNO is with well qualified professional people and having experience more than 30 years in the Pharmaceutical Industry focusing on critical Pharmaceutical and Biotech industry functions, including India & Overseas markets.
SHLOK TECHNO goes beyond being formal business associates; beyond just getting the job done; beyond the robotic process of problem solving. By melding service with strategy, we create solutions that create value.

-
Qualification :
- - M.Sc. (Organic Chemistry),
- - MBA (Pharmaceutical Marketing),
- - DIS (Post Diploma in Industrial safety). Experience :
- - 30+ Years in Pharmaceutical Manufacture
ABILITIES:
- Designing, installation and commissioning of API Manufacturing Plant.
- To acquire all necessary government licenses to manufacture API.
- To design & establish DSIR approved R&D centre for development of API.
- To review and approve TTD (Technology Transfer Document) for Biotech & Synthetic API, and ensure the Technology at PILOT PLANT. The successful transfer of Technology to commercial scale up batches and assimilates the process and other aspects thoroughly while implementation of new products / technologies at the plant for optimum output and quality standards.
- Strong hold on designing and implementation of Quality Systems and Regulatory compliances (i.e. To review Qualifications for Water System, HVAC, Production & utility equipments, Manufacturing and Testing Process Validations , Annual Product Quality Review ( APQR ).To review technology transfer documents, Product Development Report, Drug Master Formula (DMF), to review specifications of Raw Materials, Packing Materials, water and Finished Goods, to review Standard Operation Procedures ( SOPs), to review Cleaning Validation and Equipment Qualification Protocol and planning, to handle deviations, change controls and their evaluations, to handle Out of Specifications and their investigations, to handle market complains and recalls, to review for internal self audit, to conduct training on GMP and Quality System.
- Working as an auditor in international prestigious inspections like WHO, EDQM, USFDA, and OHSAS 18001 (inspection of Health and Safety).
- FDA Approved by Govt. of Maharashtra and Gujarat in Bulk drug Production, Approval no: TP/GTB/3II/1098/46468/B and JC/KD/QP-172/157-09/3.
- Highly exposed to the high-tech computerized process operating systems like DCS, SCADA, and PLC.
- Over all techno-commercial experience in Pharmaceutical Industries in various functions like Marketing, Planning and implementations of manufacturing operations ( Biotech & Synthetic) in API and its formulations, Designing Quality Systems for water, air and finished goods and their implementation, Designing cost awareness programs and evaluations of their implementations, Designing HR policies for competent personnel and their regular training, Liaison with government authorities like FDA, Pollution Control Board, Industrial Corporation, Central & State Excise, Factory Inspectorate, etc.
RESPONSIBILITIES:
- • Over all responsible for loss and profit of API- SBU. Controlling budget by management of all departments like API Marketing , QA, QC, HRD, Accounts, Security, Stores, Production, Engineering, R&D, Safety and ETP.
- To review and approve TTD (Technology Transfer Document) for API, and ensure the Technology at PILOT PLANT. The successful transfer of Technology to commercial scale up batches and assimilates the process and other aspects thoroughly while implementation of new products / technologies at the plant for optimum output and quality standards.
- Authorized Signatory for correspondence with government bodies like FDA, Pollution Control Board, Excise, Industrial Corporation, Explosive License Authorities, etc.
- The major responsibility is to work for “Best Quality” and to strive for continuous improvement in GMP activities to achieve organizational goal, optimum output and quality standards.
- To forecast, plan, control and review total production and provide MIS incase of deviations from the planning.
- To strive for continuous improvement in stock turns, process consistency, first time right deliverance, on time in full delivery, adherence to reduction of wastages (reprocessing, unplanned break downs, re-packaging, overtime, excess reflux rates, storage of inventory, movement of material between manufacturing stages), and reduction in customer complaints.
- To ensure accurate and timely indents for raw materials, and availability of raw materials in premises as per planning and inform superior incase of deviation.
- To make sure that all production deviations are reported and evaluated and that critical deviations are investigated and the conclusions are recorded.
- To make sure that the validation protocols are prepared, reviewed and approved.
- Implementation of CGMP at plant along with QA department and training of plant personnel on SOPs, documentation and CGMP compliances as per ICH Q7a guidelines and other regulatory requirements.
- To ensure compliance conforming to ISO 14001 and OHSAS 18001 norms.
- To make sure that HAZOP studies are done for all operations likely to have risk implication to men, equipments and business, in co-ordination with EHS Dept.
- To approve the specifications / requirements given by operations group to the Project department for capacity enhancement, modifications and new products.
- To lead the Marketing Department for chasing the sales target and to understand the requirement of market.
- To assure good HR policies and systems for plant, to keep in tune with changing environments for retaining employees and team building processes.
- To carryout periodic appraisal of the group personnel for their merit rating and participate in the recruitment, selection training and development of employees.
- To ensure strict adherence towards Technology Security Systems developed to control leakage of confidential information.